MEDICAL POWER SUPPLIES

The medical field for switching power supplies is a very demanding application but at the same time is the best challenge for the skilled designers, the R&D have to define the best solution that balance the typical compromises of these devices, a complex challenge where the experience over the years is fundamental to success and can make the difference in the strict norms requirements.
The limited space in the medical devices and therefore the compact dimensions are essential Example XP Power's GCS, but this is in conflict to the thermal management of the power supply.
Normally the need is to cool with minimum air flow and often, in the most extreme solutions, the request is to have natural convection cooling; in addition the custmer requirements may have high power levels and in environments where the temperature level can reach critical values for some main components . Ex. XP Power 's CCL400 & TDK-Lambda's CUS150M.
The search for compact dimensions also impact negatively the levels of electromagnetic emissions and reduce their margins compared to the standards set by the regulations; emission levels that are mitigated by increasingly refined conversion topologies to limit the "weight" of the filters, which in turn impacts on the leakage currents, even those with norms limits. Ex.TDK-Lambda's QM
In some cases it is necessary to provide for the use of Class II solutions, without earth protection, thus drastically reducing leakage currents
No doubt of electric safety is a crucial point of design for this specific applications, this point increase heavily the specifications required for industrial applications adding to has already been introduced.
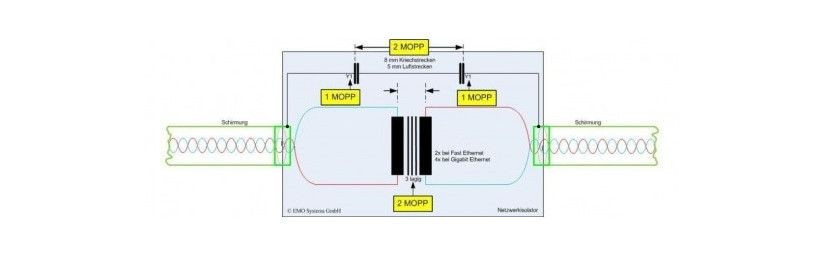
The design of the insulation with double protection barriers 2xMoPP and 2xMoOP (Means of Patient / Operator Protection) respectively Patient protection and Operator simplifies the safe integration of the power supply and the compliance of the complete medical device to the regulations for both case of use with or without applied parts to the patient.
The standard IEC / EN / UL60601 define the voltage levels for the protection barriers and the distances in air and surface (Cleareance / Creepage) in the basic and double / reinforced type, usually the PS manufacturer provides the insulation scheme of the power supply to reduce timing in the complete device certification.
Moreover it establishes the maximum permissible leakage current (Leakage) under normal conditions (NC) and single fault conditions (SFC).
The third edition (Ed. 3) introduces the risk management and the implementation of a risk management procedure according to ISO14971.
The new edition (Ed.4) improve requirements for immunity of the equipment, mainly due to the proliferation of wireless communication devices that operate within the local proximity of what can essentially lead to serious injury of the patient.
These wireless devices can be for example mobile phones, bluetooth devices, WiFi, RFID.
It also introduces a risk analysis element (risk managment) in deciding which levels of immunity are appropriate for the equipment, its intended operating environment and predictable levels of disturbances. This is due to the inclusion in the standard of equipment designed to operate outside hospital environments where there is less supervision of the equipment and less control over the electromagnetic field. Part of the risk approach is that manufacturers need to be clear about the essential operation of their product and mitigate the risk of failures or abnormal or unexpected operations by choosing appropriate levels of immunity.
The ISO 13485: 2016 is the most appropriate quality management system for medical device manufacturers to be comply with; certification of ability to provide medical applications devices that consistently meet the regulatory safety aspects, involves the design and development of production, storage and distribution.
XP Power
The mission critical nature of medical devices demands high quality, reliable and safe products. Our goal is to consistently deliver products that meet this criteria and to ensure that we meet this goal XP Power factories are certified to medical Quality Manufacturing System ISO13485 and all of our products are designed to rigorous standards as well as undergoing extensive testing. We use DFMEA (Design Failure Mode Effects Analysis), PFMEA (Process Failure Mode Effects Analysis) and ISO14971 (risk management for medical devices) to ensure out products are as reliable and safe as possible.?
TDK-Lambda
ISO 13485:2003 and EN ISO 13485:2012 certification has been awarded to TDK-Lambda UK for its comprehensive quality management system for the design, manufacture, procurement, sales, distribution, product support and service of switch mode power supplies for medical applications.